A. Aprile, G. Palermo, A. De Luca, R. Pinalli, E. Dalcanale and P. Pagliusi
1- CNR-NANOTEC Istituto di Nanotecnologia and Department of Physics, University of Calabria, 87036-Rende, Italy.
2- Department of Chemistry, Life Sciences and Environmental Sustainability & INSTMRU Parma, Parco delle Scienze 17/A, 43124 Parma, Italy
Abstract
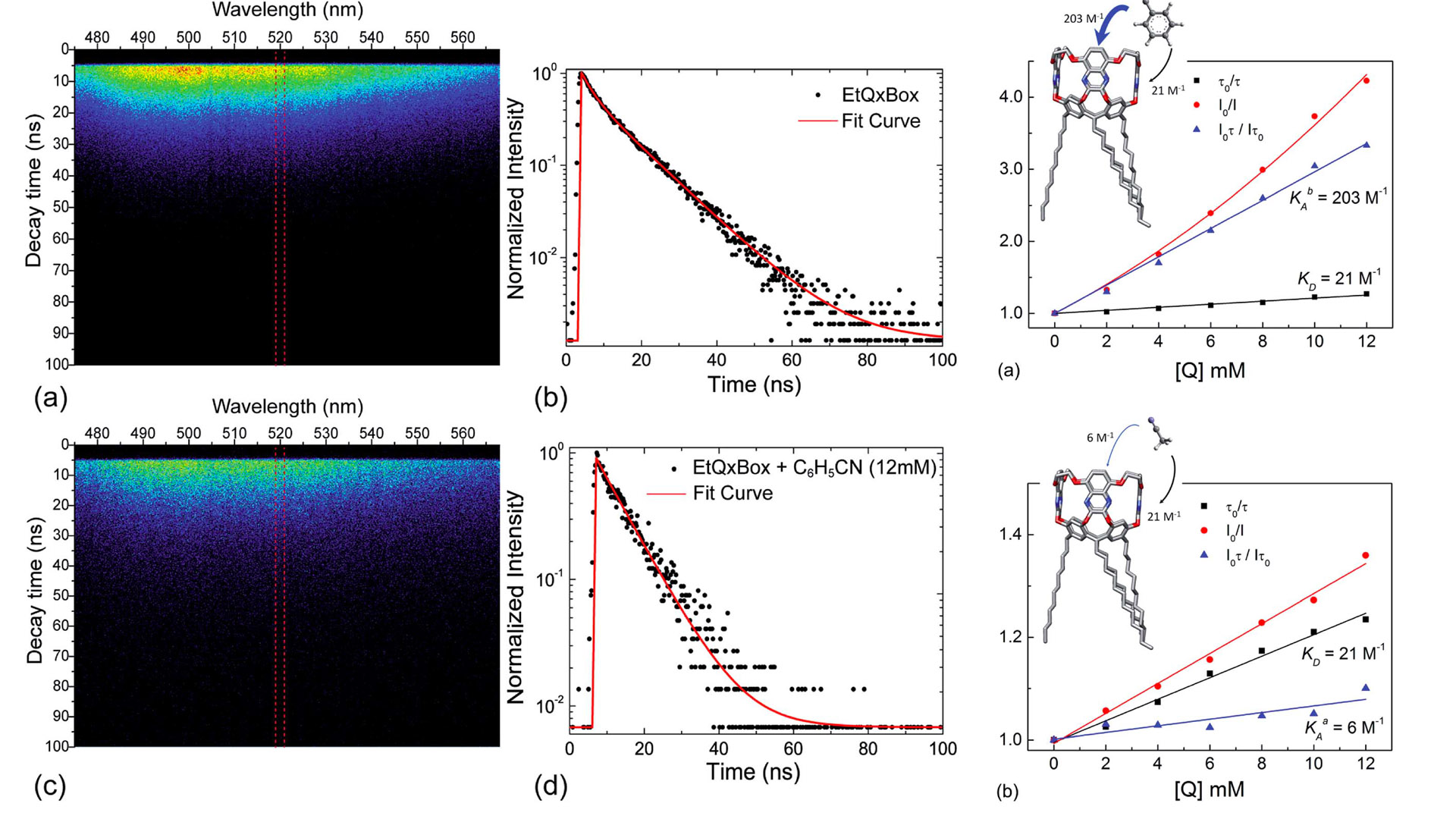
Reliable chemical sensors with high selectivity and sensitivity toward specific target molecules require rational synthesis of receptors, in-depth characterization of their complexation abilities and highly efficient transduction of the molecular recognition event. Here we report a steady-state and time-resolved fluorescence investigation of EtQxBox, a fluorescent conformationally blocked quinoxaline-based cavitand, aimed at assessing its selectivity toward aromatic versus non-aromatic analytes in solution. Fluorescence quenching of the EtQxBox in acetone is observed at increasing concentration of both aromatic (i.e. benzonitrile) and aliphatic (i.e. acetonitrile) compounds. The combination with fluorescence lifetime measurements permits to discriminate the predominantly static quenching of the aromatic analyte, due to non-fluorescent host–guest complex formation, from the mostly dynamic quenching of the non-aromatic compound, resulting from aspecific diffusive collisions between the fluorophore and the quencher. The equilibrium association constants for both the complexes have been estimated using Stern–Volmer model.